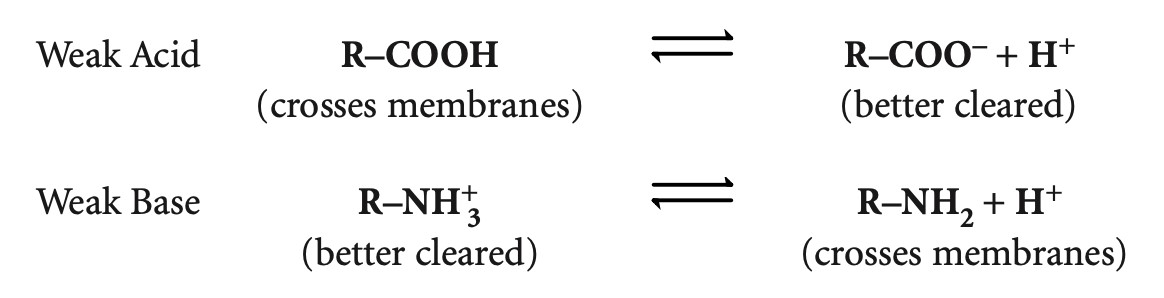Pharmacokinetics
Pharmacokinetics describes the effects of the body on drugs, e.g., absorption, distribution, metabolism, excretion, etc. simple definition for pharmacokinetics would be what the body does to the drug.
Pharmacodynamics
Pharmacodynamics denotes the actions of the drug on the body, such as mechanism of action and therapeutic and toxic effects.
Permeation
Permeation is the movement of drug molecules into and within the biologic environment, Permeation is simply meaning the ability of drug to move effectively in the body.
factors that influencing permeation:
-
Lipid solubility
Ability to diffuse through lipid bilayers (lipid solubility) is important for most drugs; however, water solubility can influence permeation through aqueous phases. Lipid diffusion is the most important limiting factor for drug permeation because of the large number of lipid barriers that separate the compartments of the body.
-
Concentration gradient
Most drugs Diffusion down a concentration gradient—only free, unionized drug forms contribute to the concentration gradient.
-
Surface area and vascularity
Important with regard to absorption of drugs into the systemic circulation. The larger the surface area and the greater the vascularity, the better is the absorption of the drug. For instance, when you orally administered drug if we just only think about surface area, is the drug more likely to absorbed in stomach or small intestine? It’s going to be small intestine because it has greater surface area. Another example applied here for vascularity, if we inject drug intramuscularly versus subcutaneously, which one of these would have faster rate of absorption? The answer is intramuscular injection because muscles are more vascular.
There are also four common processes in the movement of drug through membrane (permeation):
-
Aqueous diffusion (filtration)
This is for the diffusion of water soluble molecules, Occurs within the larger aqueous compartments of the body and across epithelial membrane tight junctions and endothelial lining of blood vessels through aqueous pores, aqueous diffusion driven by the concentration gradient of the permeating drug, Drug molecules that are bound to large plasma proteins (e.g., albumin) do not permeate most vascular aqueous pores.
-
Lipid diffusion
This is for the diffusion of lipid soluble molecules, furthermore, lipid diffusion is the most important limiting factor for drug permeation because of the large number of lipid barriers that separate the compartments of the body. Determined by: aqueous partition coefficient, concentration difference, ionization of weak acid and weak base (can be estimated by pKa and pH).
-
Carrier transport
Drugs that do not readily diffuse through membranes may be transported across barriers by mechanisms that carry similar endogenous substances.
-
Endocytosis and exocytosis
Endocytosis occurs through binding of the molecule to specialized components (receptors) on cell mem- branes, with subsequent internalization by infolding of that area of the membrane. The contents of the resulting intracellular vesicle are subsequently released into the cytoplasm of the cell.
Ionization
Many drugs are weak acids or weak bases and can exist in either nonionized or ionized forms in an equilibrium, depending on the pH of the environment and the pKa (the pH at which the molecule is 50% ionized and 50% nonionized), Only the nonionized (uncharged) form of a drug crosses membranes. The ionized form is better renally excreted because it is water soluble.
Simply we can say:
Ionized = water soluble
Nonionized = lipid soluble
Weak acids: aspirin, penicillin, cephalosporins, loop and thiazide diuretics.
Weak bases: morphine, local anesthetics, amphetamines, PCP.
The nonionized form of acids is when carboxylic group(hydrogen) attaches to it. R-COOH, it has no charge, this is when we call it nonionized form, so it easily crosses the membrane. When we remove that hydrogen now, we give weak acids negative charge indicates the ionized form of the drug, so it better cleared from the body, because it is more water soluble.
Weak base it has hydrogen on it, so it is ionized and better cleared from the body. when you remove the hydrogen from the base, the base drug has no charge and is nonionized, so, it crosses membrane better.
In acidic environment weak acids stick to the hydrogen and stayed in nonionized forms, but in basic environment they just got deprotonated (losing the hydrogen) and become ionized.
In acidic environment weak bases stick to hydrogen and stayed ionized, but in basic environment they lose hydrogen and become nonionized.
Ionization Increases Renal Clearance of Drugs
Only free, unbound drug is filtered.
Both ionized and nonionized forms of a drug are filtered.
Only nonionized forms undergo active secretion and active or passive reabsorption.
Ionized forms of drugs are “trapped” in the filtrate.
Acidification of urine → increases ionization of weak bases → increases
renal elimination.
Alkalinization of urine → increases ionization of weak acids → increases renal elimination.
To Change Urinary pH
Acidify: NH4Cl, vitamin C, cranberry juice
Alkalinize: NaHCO3, acetazolamide (historically)
ABSORPTION:
Concerns the processes of entry of a drug into the systemic circulation from the site of its administration.
The determinants of absorption are those described for drug permeation. Absorption always happen when there are 2 compartments, movement from one compartment to the blood.
Intravascular administration (e.g., IV) does not involve absorption because it is directly into the blood, and there is no loss of drug. Bioavailability = 100%
What is the most rapid route of absorption? The answer is inhalation.
With extravascular administration (e.g., per os [PO; oral], intramuscular [IM], subcutaneous [SC], inhalation), less than 100% of a dose may reach the systemic circulation because of variations in bioavailability.
Join Art of Medics
Become Part of Art of Medics for further scientific purposes