Amino acids
Amino acids are building blocks of proteins. There have been 300 different amino acids found world widely. However, proteins in mammalian bodies (including human body) made up of with 20 different amino acids. All amino acids have a central carbon atom attached to a carboxyl group, an amino group, and a hydrogen atom. The amino acids differ from one another only in the chemical nature of the side chain (R). The amino and carboxy groups of amino acids can react in a head-to-tail fashion, eliminating a water molecule and forming a covalent amide linkage, which in the case of peptide and proteins, is typically referred to a peptide bond. 20 L-α-amino acids found in mammalian proteins. They are optical active (chiral). The L and D isomers of amino acids. The L and D isomers are mirror images of each other.
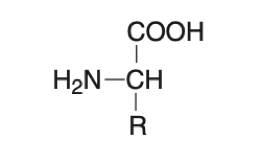
Figure 1. structure of amino acids
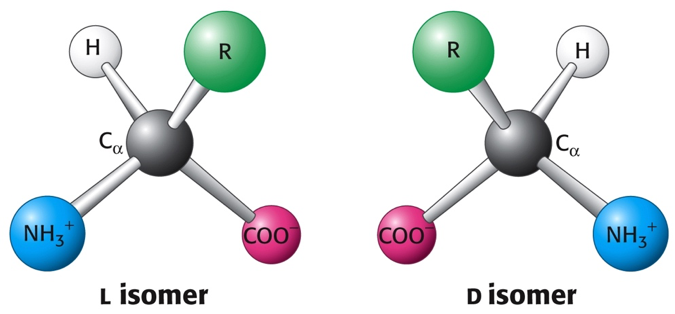
Figure 2. The L and D isomers of amino acids
Classification of amino acids
Classification of amino acids is not complicated, amino acids are hydrophobic (water phobia) or hydrophilic (like water). When proteins fold, amino acids with hydrophobic side chains are in the interior of the molecule where they are protected from water and those with hydrophilic side chains are on the surface.
Hydrophobic amino acids
Phenylalanine and tyrosine are precursors for catecholamines.
Tryptophan can form serotonin and niacin.
Valine, leucine, and isoleucine are branched-chain amino acids whose metabolism is abnormal in maple syrup urine disease.
Proline is a secondary amine whose presence in a protein disrupts nor- mal secondary structure.
Figure below is the list of hydrophobic amino acids.
Figure 3. hydrophobic amino acids
Hydrophilic amino acids
Hydrophilic amino acids have side chains that contain O or N atoms. Some of the hydrophilic side chains are charged at physiologic pH. The acidic amino acids (aspartic and glutamic acids) have carboxyl groups that are negatively charged, whereas the basic amino acids (lysine, arginine, and histidine) have nitrogen atoms that are positively charged. Here is the list of hydrophilic amino acids.
Figure 4. The Hydrophilic Amino Acids
Abbreviations and symbols for amino acids
three-letter abbreviation and one-letter symbol
unique first letter: I= isoleucine
most commonly occurring amino acids have priority: G=glycine, not Glu or Gln
similar sounding names: F=phenylalanine (Phe), W=tryptophan(“twyptophan”)
letter close to initial letter: asparate = Asp = D (near A), Lysine = Lys = K (near L)
Figure 5. Abbreviations and symbols for amino acids
Essential Amino Acids
All 20 types of amino acids are required for protein synthesis. These amino acids can be derived from digesting dietary protein and absorbing their constituent amino acids or, alternatively, by synthesizing them de novo.
The 10 amino acids listed here in the figure below cannot be synthesized in humans and therefore must be provided from dietary sources. These are called the essential amino acids. Arginine is required only during periods of growth, or positive nitrogen balance.
Figure 6. Essential amino acids
Join Art of Medics
Become Part of Art of Medics for further scientific purposes

