- Chapter 4-Carbon and the Molecular Diversity of Life
1) The element present in all organic molecules is .
A) hydrogen
B) oxygen
C) carbon
D) nitrogen
Answer: C
2) The complexity and variety of organic molecules is due to .
A) the chemical versatility of carbon atoms
B) the variety of rare elements in organic molecules
C) the diverse bonding patterns of nitrogen
D) their interaction with water
Answer: A
3) The experimental approach taken in current biological investigations presumes that .
A) simple organic compounds can be synthesized in the laboratory from inorganic precursors, but complex organic compounds like carbohydrates and proteins can be synthesized only by living organisms
B) a life force ultimately controls the activities of living organisms and this life force cannot be studied by physical or chemical methods
C) living organisms are composed of the same elements present in nonliving things, plus a few special trace elements found only in living organisms or their products
D) living organisms can be understood in terms of the same physical and chemical laws that can be used to explain all natural phenomena
Answer: D
4) Differences among organisms are caused by differences in the .
A) elemental composition from organism to organism
B) types and relative amounts of organic molecules synthesized by each organism
C) sizes of the organic molecules in each organism
D) types of inorganic compounds present in each organism
Answer: B
5) Stanley Miller’s 1953 experiments supported the hypothesis that .
A) life on Earth arose from simple inorganic molecules
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth
C) life on Earth arose from simple organic molecules, with energy from lightning and volcanoes
D) the conditions on early Earth were conducive to the origin of life
Answer: B
6) When Stanley Miller applied heat and electrical sparks to a mixture of simple inorganic compounds such as methane, hydrogen gas, ammonia, and water vapor, what compounds were produced?
A) only simple organic compounds such as formaldehyde and cyanide
B) mostly hydrocarbons
C) only simple inorganic compounds
D) simple organic compounds, amino acids, and hydrocarbons
Answer: D
7) Which of the following is true of carbon?
A) It forms only polar molecules.
B) It can form a maximum of three covalent bonds with other elements
C) It is highly electronegative.
D) It can form both polar and nonpolar
Answer: D
8) Why is carbon so important in biology?
A) It is a common element on Earth.
B) It has very little electronegativity, making it a good electron donor.
C) It bonds to only a few other eleme
D) It can form a variety of carbon skeletons and host functional groups
Answer: D
9) How many electron pairs does carbon share to complete its valence shell?
A) 2
B) 3
C) 4
D) 8
Answer: C
10) A carbon atom is most likely to form what kind of bond(s) with other atoms?
A) ionic
B) hydrogen
C) covalent
D) ionic bonds, covalent bonds, and hydrogen bonds
Answer: C
11) Why are hydrocarbons insoluble in water?
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkage
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkage
C) They exhibit considerable molecular complexity and diversity.
D) They are less dense than water.
Answer: B
12) Which of the following statements correctly describes cis-trans isomers?
A) They have variations in arrangement around a double bond.
B) They have an asymmetric carbon that makes them mirror image
C) They have the same chemical properties.
D) They have different molecular formula
Answer: A
13) Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that .
A) have identical chemical formulas but differ in the branching of their carbon skeletons
B) are mirror images of each other
C) differ in the location of their double bonds
D) differ in the arrangement of atoms around their double bonds
Answer: B
14) What determines whether a carbon atom’s covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration?
A) the presence or absence of bonds with oxygen atoms
B) the presence or absence of double bonds between the carbon atom and other atoms
C) the polarity of the covalent bonds between carbon and other atoms
D) the solvent in which the organic molecule is dissolved
Answer: B
15) Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, a hydrocarbon chain with the same number of carbon atoms, but with one or more double bonds, will .
A) be more flexible in structure
B) be more constrained in structure
C) be more polar
D) have more hydrogen atoms
Answer: B
16) Organic molecules with only hydrogens and five carbon atoms cannot _ .
A) have a branching carbon skeleton
B) have different combinations of double bonds between carbon atoms
C) have different positions of double bonds between carbon atoms
D) form enantiomers
Answer: D
17) The two molecules shown in the figure below are best described as_ .
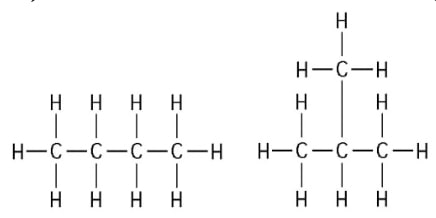
A) enantiomers
B) structural isomers
C) cis-trans isomers
D) chain length isomers
Answer: B
18) The figure above shows the structures of glucose and fructose. These two molecules differ in the .
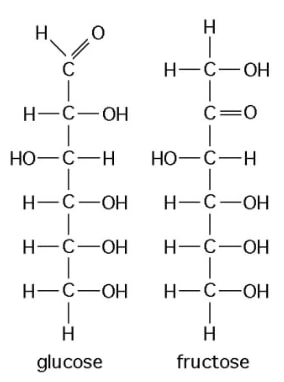
A) number of carbon, hydrogen, and oxygen atoms
B) types of carbon, hydrogen, and oxygen atoms
C) arrangement of carbon, hydrogen, and oxygen atoms
D) number of oxygen atoms joined to carbon atoms by double covalent bonds
Answer: C
19) The figure above shows the structures of glucose and fructose. These two molecules are .
A) isotopes
B) enantiomers
C) cis-trans isomers
D) structural isomers
Answer: D
20) The two molecules shown in the figure below are best described as .
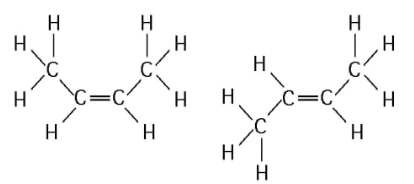
A) enantiomers
B) radioactive isotopes
C) structural isomers
D) cis-trans isomers
Answer: D
21) Which of the following illustrations is NOT a structural isomer of an organic compound with the molecular formula C6H14? For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted.
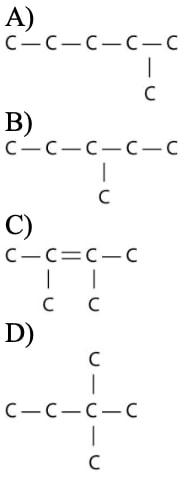
Answer: C
22) Which of the pairs of molecular structures shown below depict enantiomers (enantiomeric forms) of the same molecule?
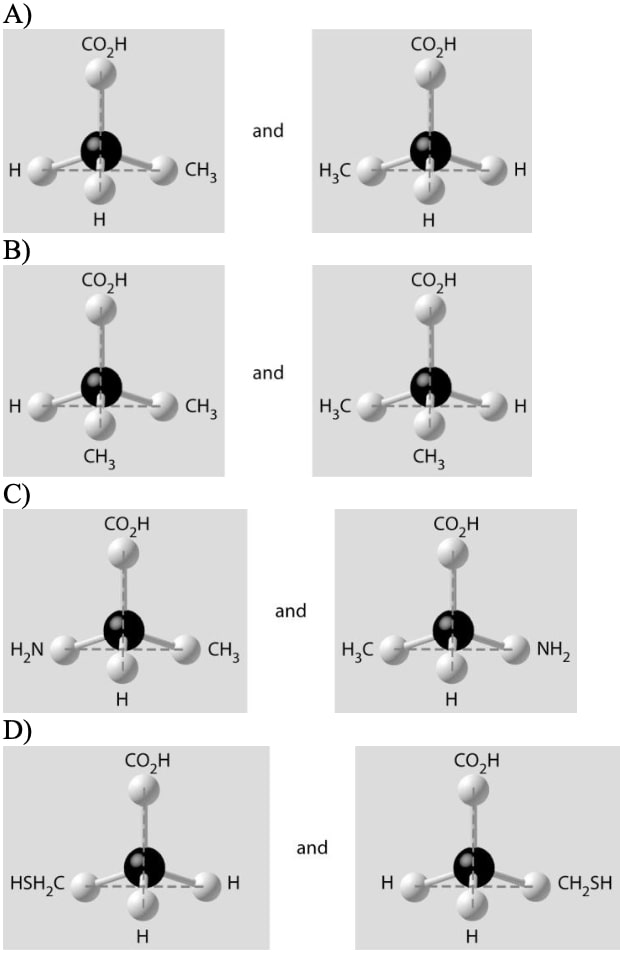
Answer: C
23) Which of the pairs of molecular structures shown below do NOT depict enantiomers (enantiomeric forms) of the same molecule?
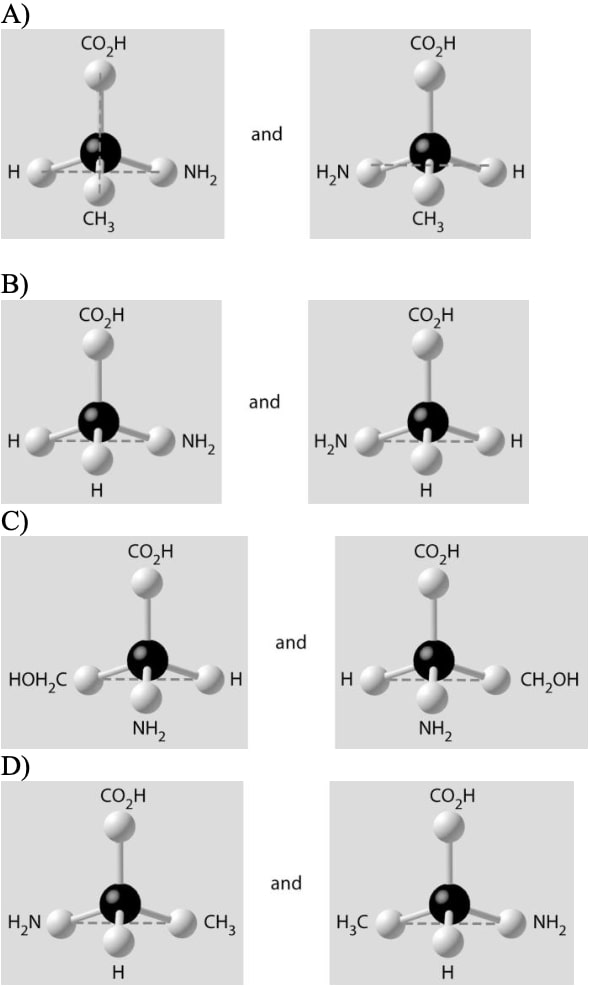
Answer: B
24) Thalidomide and L-dopa, shown below, are examples of pharmaceutical drugs that occur as enantiomers, or molecules that .
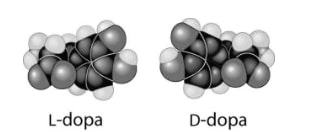
A) have identical three-dimensional shapes
B) are mirror images of one another
C) are mirror images of one another and have the same biological activity
D) are cis-trans isomers
Answer: B
25) Which of the following molecules is polar?
C3H7OH C2H5COOH
A) C3H7OH and C2H5COOH are both polar molec
B) Neither C2H5COOH or C3H7OH is polar.
C) C2H5COOH is polar, but C3H7OH is not pola
D) C3H7OH is not polar, but C3H7OH is polar.
Answer: A
26) Which of the functional groups below acts most like an acid in water?
A) amino
B) carbonyl
C) carboxyl
D) hydroxyl
Answer: C
27) A compound contains hydroxyl groups as its predominant functional group. Therefore, this compound .
A) lacks an asymmetric carbon and is probably a fat or lipid
B) should dissolve in water
C) should dissolve in a nonpolar solvent
D) will not form hydrogen bonds with water
Answer: B
28) Which two functional groups are always found in amino acids?
A) carbonyl and amino groups
B) carboxyl and amino groups
C) amino and sulfhydryl groups
D) hydroxyl and carboxyl groups
Answer: B
29) Amino acids are acids because they always possess which functional group?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
Answer: C
30) A hydrocarbon skeleton is covalently bonded to an amino group at one end and a carboxyl group at the other end. When placed in water this molecule would function .
A) only as an acid because of the carboxyl group
B) only as a base because of the amino group
C) as an acid and a base
D) as neither an acid nor a base
Answer: C
31) Which chemical group can act as an acid?
A) amino
B) carbonyl
C) carboxyl
D) methyl
Answer: C
32) Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other? Testosterone and estradiol .
A) are structural isomers but have the same molecular formula
B) are cis-trans isomers but have the same molecular formula
C) have different functional groups attached to the same carbon skeleton
D) are enantiomers of the same organic molecule
Answer: C
33) What is the name of the functional group shown in the figure below?
A) carbonyl
B) ketone
C) aldehyde
D) carboxyl
Answer: D
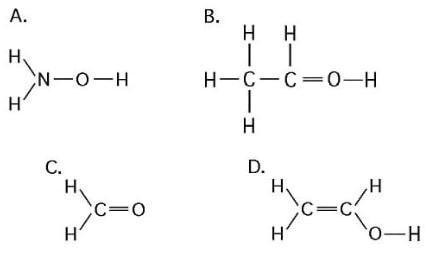
34) Which of the structures illustrated above is an impossible covalently bonded molecule?
A) A
B) B
C) C
D) D
Answer: B
35) In which of the structures illustrated above are the atoms bonded by ionic bonds?
A) A
B) B
C) C
D) none of the structures
Answer: D
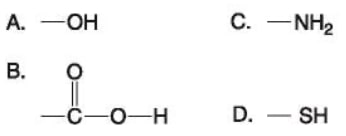
36) Which functional group shown above is characteristic of alcohols?
A) A
B) B
C) C
D) D
Answer: A
37) Which functional group(s) shown above is (are) present in all amino acids?
A) A and B
B) B and D
C) C only
D) B and C
Answer: D
38) Which of the groups shown above is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?
A) A
B) B
C) C
D) D
Answer: D
39) Which of the groups above is a carboxyl functional group?
A) A
B) B
C) C
D) D
Answer: B
40) Which of the groups above is an acidic functional group that can dissociate and release H+
into a solution?
A) A
B) B
C) C
D) D
Answer: B
41) Which of the groups above is a basic functional group that can accept H+ and become positively charged?
A) A
B) B
C) C
D) D
Answer: C
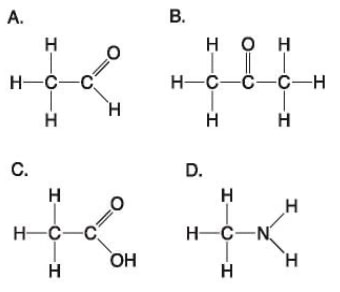
42) Which molecule shown above would have a positive charge in a cell?
A) A
B) B
C) C
D) D
Answer: D
43) Which molecule(s) shown above is (are) ionized in a cell?
A) A
B) B and D
C) C and D
D) D
Answer: C
44) Which molecules shown above contain a carbonyl group?
A) A and B
B) B and C
C) B, C, and D
D) C and D
Answer: A
45) Which molecule shown above has a carbonyl functional group in the form of a ketone?
A) A
B) B
C) C
D) D
Answer: B
46) Which molecule shown above has a carbonyl functional group in the form of an aldehyde?
A) A
B) B
C) C
D) D
Answer: A
47) Which molecule shown above contains a carboxyl group?
A) A
B) B
C) C
D) D
Answer: C
48) Which molecule shown above can increase the concentration of hydrogen ions in a solution and is, therefore, an organic acid?
A) A
B) B
C) C
D) D
Answer: C
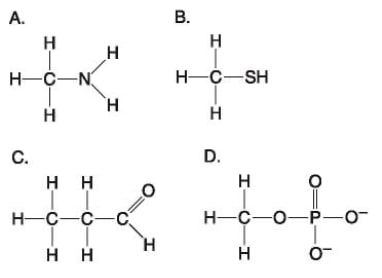
49) Which molecule shown above can form a cross linkage?
A) A
B) B
C) C
D) D
Answer: B
50) Which molecule shown above contains an amino functional group, but is NOT an amino acid?
A) A
B) B
C) C
D) D
Answer: A
51) Which molecule shown above is a thiol?
A) A
B) B
C) C
D) D
Answer: B
52) Which molecule shown above contains a functional group that cells use to transfer energy between organic molecules?
A) A
B) B
C) C
D) D
Answer: D
53) Which molecule shown above can function as a base?
A) A
B) B
C) C
D) D
Answer: A
54) Which of the following is a FALSE statement concerning amino groups? Amino groups
.
A) are basic with respect to pH
B) are found in amino acids
C) contain nitrogen
D) are nonpolar
Answer: D


